Mesoporous Metal Oxides, Metal Sulfides and Metal Selenides
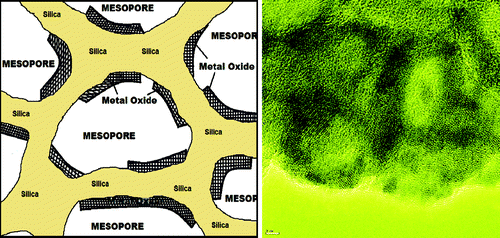
We employed the salts-surfactant liquid crystalline (LLC) mesophases to design mesoporous thin films. The salt surfactant mesophases can be dissolved in various solvents and spin or dip coated over any substrate to obtain LLC thin films. If one introduces a polymerizing sol-gel precursor to this media, such as TMOS or TTB one can also obtain the solid thin films. In this assembly process, we use two surfactants and a salt LLC mesophases that can accommodate more salt in the media. We also use two solvents, one is a common solvent, such as water or ethanol, and the other is a salt in its molten phase. Water or alcohol completely evaporates after spin coating and it is very important to obtain a homogeneous clear solutions. However the second solvent never evaporates and holds the mesophases after spin coating and also necessary for further reaction during the calcination step. The process is called molten salt assisted self-assembly (MASA). MASA is a new and also an effective method to produce mesoporous thin films composed of silica-metal oxide and metal titanates. In the silica case, one can obtain mesoporous silica with a fully metal oxide coated pore system. The amount of metal oxide can be more than silica. The metal oxide coating is very uniform and it can be as thin as less than 2.0 nm. These oxides are very reactive towards hydrogen sulfide and hydrogen selenide gases and can be converted to metal sulfides and metal selenides.
MASA is an important self-assembly process that was introduced to the literature from our group. If one uses a titanium alkoxide as the polymerizing agent, one can produce metal titanates. We are currently working on range of metal titanates to prove the concept and use these thin films as electrodes in various energy applications. The method can be expanded include other gol-gel precursor. Some our paper are listed here as examples.
Publications: Some recent examples
In this article, we established a new synthetic method called MASA that uses two solvents that are ethanol and a metal nitrate salt. The method can be used to produce transparent mesoporous metal titantes and metal sulfide-titania, metal selenide-titania coupled semiconductors that can be used for solar energy applications.
(a and b) XRD pattern of meso-Zn2TiO4 (with a Zn/Ti mole ratio of 0.86), annealed at different temperatures as marked on the patterns.
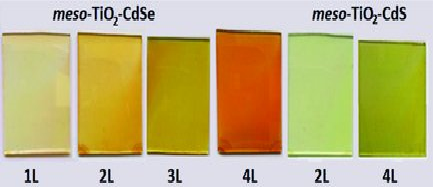
Mesoporous silica metal oxide (ZnO and CdO) thin films have been used as metal ion precursors to produce the first examples of mesoporous silica metal sulfide (meso-SiO2@ZnS, meso-SiO2@CdS) or silica metal selenide (meso-SiO2@ZnSe, meso-SiO2@CdSe) thin films, in which the pore walls are made up of silica and metal sulfide or metal selenide nanoflakes, respectively.A gentle chemical etching with a dilute HF solution of the meso-SiO2@CdS (or meso-SiO2@CdSe) produces mesoporous cadmium sulfide (meso-CdS) (or cadmium selenide, meso-CdSe). Surface modified meso-CdS displays bright blue photoluminescence upon excitation with a UV light. The mesoporous silica metal oxides are formed as metal oxide nanoislands over the silica walls through a self-assembly process of a mixture of metal nitrate salt-two surfactants-silica source followed by calcination step.